New data presented at ATTD demonstrates the system's ability to help people with type 1 diabetes exceed international targets for outcome measures
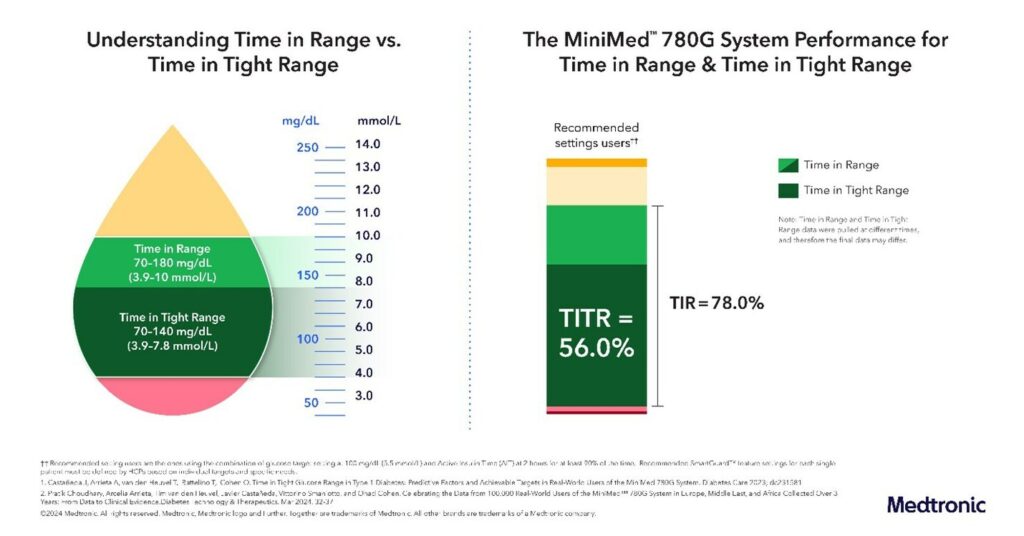
dublin and florence, italy, March 9, 2024 /PRNewswire/ — Medtronic, Inc. (NYSE: MDT), a global leader in healthcare technology, today shared a powerful new set of clinical and real-world evidence for the MiniMed™ 780G System around the world. This also includes the largest dataset from early users. US. The data was released on the 17th.th International Conference on Advanced Technologies and Treatments of Diabetes (ATTD) florence, italy. These results are based on his three years of data published in. Diabetes technology and treatment Over 100,000 real-world users achieved a 78% time-to-reach (TIR) using the recommended optimal settings, exceeding the international goal of 70% TIR.
Time in range is the percentage of time a diabetic patient spends between 70 and 180 mg/dL (3.9 and 10.0 mmol/L). Narrow range time is a new metric defined as the percentage of time diabetics spend in a narrower range of 70 to 140 mg/dL (3.9 to 7.8 mmol/L), which more tightly lowers the upper limit of blood sugar levels. Reflects blood sugar levels in people without diabetes (also called normoglycemia). A MiniMed™ 780G system user (n = 13,461) achieved his TITR of >56% using the recommended optimal settings (target set to 100 mg/dL with 2 hours effective insulin time) .
The new data is aimed at evaluating the ability of the MiniMed™ 780G system to help users achieve Time in Tight Range (TITR) goals, a new It is a supplementary indicator that more closely reflects blood sugar levels in individuals without diabetes. Also called euglycemia or normoglycemia, it is defined as the percentage of time a person spends in her 70-140 mg/dL glucose range. TITR lowers the time-in-range upper threshold from 180 mg/dL to 140 mg/dL. The results show that a user (n = 13,461) achieved his TITR by over 56% using the recommended optimal settings (target set to 100 mg/dL with effective insulin time of 2 hours). I showed it. This data adds further evidence that her TITR goal of 50% or higher is a reasonably achievable goal with appropriate treatment options.
“Since the landmark DCCT study, numerous retrospective studies have demonstrated an association between increased flight time and decreased diabetic complications.1-12 There is no question that elevated blood sugar levels are harmful, and the average blood sugar levels in people with type 1 diabetes exceed what is clinically acceptable,'' said Robert Bigarsky, M.D., chief medical officer, Medtronic Diabetes. Ta. Randomized controlled trials and real-world studies show that the MiniMed™ 780G System far exceeds international goals, maximizes reach, and goes one step beyond by moving people closer to euglycemia. .13,14 In the absence of a cure, our goal is to continually innovate treatments to maximize people's health without increasing burden, and that is demonstrated with our latest AID system. I am. ”
mini med™ Early success of 780G system in the US
In the oral presentation, Dr. james thrasherM.D., founder of the Arkansas Diabetes and Endocrine Center, shared data on early real-world users (n=7,499) with type 1 diabetes of the MiniMed™ 780G system in the United States. As a result, when using the recommended optimal settings, users can achieve a TIR of 80% or more above the international blood glucose target (ADA guidelines recommend 70% of the time between 70 and 180 mg/dL). achievable and demonstrated that exits from the closed loop averaged less than once per week. The enhancements introduced in this state-of-the-art system have resulted in high levels of satisfaction and improved quality of life.15,16 In fact, the latest dQ&A US Pump Patient Survey (n=1,997) found that the MiniMed™ 780G System ranked #1 in overall pump satisfaction among pump users.*,17 The study also showed that overall satisfaction with the Guardian™ 4 sensor was comparable to competitive sensors among people with type 1 diabetes using CGM.*,18
“These results demonstrate that the MiniMed™ 780G system, when optimized at the recommended optimal settings, can help treat patients with diabetes far beyond the ADA-recommended 70% reach* goal. ,” said Dr. Thrasher. “The advent of AID systems has not only revolutionized the practice of endocrinology, but also urges all of us to implement their protective effects on overall health as early and often as possible. is the determining factor. It should be first and foremost the power of algorithms.”
About time in a narrow range
The development of continuous blood glucose monitoring has enabled the development of Time in Range (TIR), a metric currently used to determine whether patients with type 1 diabetes are meeting their glycemic control goals. . Since 2019, the goal of diabetes management has been to maintain her best TIR for as long as possible while minimizing hypoglycemia. The introduction of automated insulin dosing (AID) systems has transformed diabetes care by allowing a wider range of people to safely meet their blood sugar goals with less effort and effort. The AID system is helping people achieve more ambitious goals in glycemic control and has encouraged the emergence of new supplementary indicators that reflect blood glucose levels (euglycemia or euglycemia) in individuals without diabetes. The MiniMed™ 780G System has demonstrated that greater than 50% time in a narrow range is achievable, serving as a powerful tool for those seeking more time in euglycemic states.
About Medtronic Diabetes (www.medtronicdiabetes.com)
Medtronic Diabetes is committed to reducing the burden of diabetes by empowering individuals to live on their terms with cutting-edge diabetes technology and constant support when and how they need it. I am in charge. For more than 40 years, we are committed to pioneering first-of-its-kind innovations and designing the future of diabetes management through the power of next-generation sensors (CGMs), intelligent dosing systems, data science and AI. . Frontline customer experience.
About Medtronic
A bold idea. A bolder move. We are Medtronic.Medtronic plc, headquarters dublin, irelandis a leading global healthcare technology company that boldly challenges humanity's most challenging health problems by searching for and finding solutions. Our mission to alleviate pain, restore health and extend lifespan unites his global team of more than 95,000 passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robots, insulin pumps, surgical instruments, patient monitoring systems, and more. Driven by diverse knowledge, insatiable curiosity, and a desire to help everyone who needs it, we deliver innovative technology that changes lives every second, hour, and day. doing. Expect more from us for insight-driven care, people-first experiences, and a better outcome for the world. In everything we do, we are engineering something extraordinary. For more information about Medtronic (NYSE:MDT), visit www.Medtronic.com and follow Medtronic on LinkedIn.
Forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports filed with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
*Adults, T1, and parents of children with T1 diabetes under 18 years of age were surveyed. Individual results may vary.
source of information
- Yapanis M, James S, Craig ME, et al. Complications of diabetes and indicators of glycemic control from continuous blood glucose monitoring. J Clin Endocrinol Metab 2022;107(6):e2221–e2236
- Beck RW, Bergenthal RM, Riddlesworth TD, et al. Validation of time-in-range as an outcome measure in diabetes clinical trials. Diabetes Care 2019;42(3):400–405
- Lu J, Ma X, Zhou J et al. Association between time in range assessed by continuous glucose monitoring and diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41(11):2370–2376
- Raji R, Mishra R, Jha N, et al. Time in range measured by continuous blood glucose monitoring as a predictor of microvascular complications in type 2 diabetes: a systematic review. BMJ Open Dear Breath Care 2022;10(1):e002573
- Lu J, Ma X, Shen Y et al Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22(2):72–78
- Yoo JH, Choi MS, Ahn J et al. Associations between time in range, other core indicators, and albuminuria derived from continuous blood glucose monitoring in type 2 diabetes. Diabetes Technol Sur 2020;22(10):768–776
- Yang J, Yang X, Zhao D et al. Association between time in range assessed by continuous glucose monitoring and painful diabetic polyneuropathy. J Diabetes Invest 2021;12(5):828–836
- Hirsch IB, Shah JL, Hood KK. Connecting the dots: Validating time in range metrics with microvascular results. Diabetes Care 2019;42(3):345–348
- Mayeda L, Katz R, Ahmad I, et al. Blood glucose range and peripheral neuropathy in type 2 diabetes and chronic kidney disease. BMJ Open Diabetes Rescare 2020;8(1):e000991
- El Malahi A, Van Elsen M, Charlier S, et al. Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes. J Clin Endocrinol Metab 2022;107(2):e570–e581
- Beck RW. Association between time in range and diabetic complications: The evidence is strong. Diabetes Technol Sur 2023;25(6):375–377
- Zhu DD, Wu X, Cheng XX, et al. Time in range as a useful marker for assessing retinal functional changes in patients with diabetic retinopathy. Int J Ophthalmol 2023;16(6):915–920
- CGM and time in range. American Diabetes Association. Available at https://diabetes.org/tools-support/devices-technology/cgm-time-in-range.Accessed June 19, 2023.
- American Diabetes Association (2019). Medical standards for diabetes—2019. Diabetes Care, 42(Supplement 1): S61-S70.
- MiniMed™ 780G System SSED
- Medtronic data on file: MiniMed™780G user survey conducted in the UK from April to May 2020. Sweden, Italy, Netherlands and Belgium. N789
- dQ&A American Diabetes Panel Report; Overall Customer Satisfaction, n=146. Q4 2023: P.52 (November 2023)
- dQ&A US Diabetes Panel Report; Overall Customer Satisfaction, n=207; Q4 2023: P.85 (November 2023)
|
contact address: |
|
|
ashley patterson |
ryan weisspfenning |
|
public relations |
PR for investors |
|
+1-818-576-3025 |
+1-763-505-4626 |
SOURCE Medtronic plc